In our human vaccine business, we are advancing research and development targeting Norovirus with both oral and injectable formulations.
Norovirus Vaccine
Background
Norovirus is a causative virus of acute gastroenteritis and epidemic diarrhea, with main symptoms including nausea, vomiting, diarrhea, fever, and abdominal pain. While most cases recover within 2-3 days, it can become severe in the elderly and children. According to a report from the U.S. CDC in 2017, it is estimated that over 600 million people worldwide are infected annually, with around 200,000 deaths primarily in developing countries. As of April 2024, there are clinical trials underway for Norovirus vaccines, but no products have been approved for sale.
Norovirus is classified into seven genogroups (I-VII) based on the genetic sequence of the capsid region, with GI and GII being the main groups that infect humans. There are 9 genotypes in group GI and 22 in group GII. Among Norovirus infections worldwide, GII.4 accounts for approximately 50%.
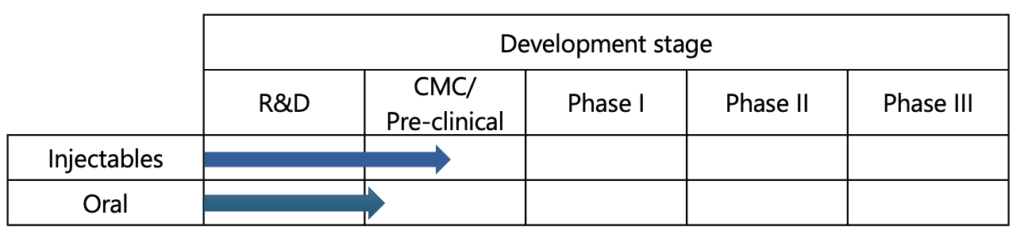
Development Status
KAICO has successfully developed antigens for nine types of Norovirus (GII.2, GII.3, GII.4, GII.6, GII.17, GI.2, GI.3, GI.4, GI.6) in collaboration with Kyushu University, with a focus on GII.4. Currently, we are working on establishing Good Manufacturing Practice (GMP) and conducting non-clinical trials to prepare for clinical drug production.
Protein Expression Services
KAICO provides “Custom Protein Expression Trials Services” using our core technology, the ‘silkworm-baculovirus system’, to supply proteins essential for vaccine development to manufacturers.
The silkworm-baculovirus expression system, which utilizes live silkworms, excels in expressing virus-like particles (VLPs) such as those of norovirus. Additionally, we have successfully expressed the spike protein of SARS-CoV-2.
For more information, please click here.



